Patient-Centricity Drives Clinical Trial Support Services Market to USD 39.4 Billion by 2032 | Rising Complexity and Outsourcing Trends
Research Reports
Jun 24, 2024
Market Overview
Clinical Trial Support Services Market Research Report Information By Service (Clinical Trial Site Management, Patient Recruitment Management [Patient recruitment & registry services, Patient retention, Others], Data Management, Administrative staff, IRB, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By Sponsor (Pharmaceutical & Biopharmaceutical, Medical Devices, Others), And By Region (North America, Europe, Asia-Pacific, And Rest Of The World) –Market Forecast Till 2032.
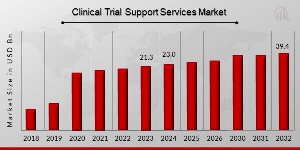
Clinical Trial Support Services Market Size was valued at USD 21.3 Billion in 2023. The viral clearance market industry is projected to grow from USD 23.0 Billion in 2024 to USD 39.4 Billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.00% during the forecast period (2024 – 2032).
Get Free Sample PDF Brochure @ https://www.marketresearchfuture.com/sample_request/22002
Market Dynamics
The Clinical Trial Support Services Market is rapidly evolving, with several key drivers influencing the demand for guiding services. A significant trend is the increasing demand for patient-centric trials, signaling a shift towards prioritizing patient experience and engagement throughout the trial process. Pharmaceutical companies are increasingly recognizing the importance of patient-centric approaches in improving recruitment, retention, and overall trial success. For example, research published in Clinical Therapeutics indicates that patient-centric trial designs can significantly enhance patient recruitment rates and reduce dropout rates, ultimately speeding up trial timelines.
Moreover, pharmaceutical companies are increasingly relying on specialized service providers to manage the growing complexity of trials. Advances in medical technology and the rise of personalized treatments have made trials more intricate, often involving complex protocols and stringent regulatory requirements. Outsourcing clinical trial operations allows companies to leverage the expertise of specialized service providers who can navigate these complexities effectively.
Furthermore, the outsourcing of clinical trial operations is driven by the need for cost-effectiveness and expertise. Pharmaceutical companies face pressure to reduce costs and accelerate drug development timelines while maintaining high standards of quality. Outsourcing enables companies to access specialized skills and resources without the overhead costs associated with in-house operations. This strategic approach not only optimizes operational efficiency but also enhances flexibility in managing clinical trial processes.
Buy Now Premium Research Report: https://www.marketresearchfuture.com/checkout?currency=one_user-USD&report_id=22002
Segment Analysis
The global clinical trial support services market is segmented based on Service, Phase, and Sponsor.
By Service: The market is segmented into Clinical Trial Site Management, Patient Recruitment Management (including patient recruitment & registry services, patient retention, and others), Data Management, Administrative Staff, IRB, and others. In 2023, the Clinical Trial Site Management segment dominated the market due to the increasing complexity of clinical trials, which require effective management of multiple sites, diverse patient populations, and stringent regulatory requirements.
By Phase: The market is segmented into Phase I, Phase II, Phase III, and Phase IV. In 2023, the Phase I category generated the most income. The growing interest in novel treatment modalities such as biologics, cell and gene therapies, and precision medicine often necessitates extensive Phase I testing to establish safety, pharmacokinetics, and preliminary efficacy.
By Sponsor: The market is segmented into Pharmaceutical & Biopharmaceutical, Medical Devices, and others. In 2023, the Medical Devices segment generated the most income. Rising healthcare expenditures, increased access to healthcare services in emerging markets, and the growing demand for advanced medical technologies worldwide are driving the expansion of the medical device market, resulting in heightened investment in clinical research to support market entry and commercialization.
Regional Analysis
The global clinical trial support services market is divided into North America, Europe, Asia-Pacific, and Rest of the World.
North America: Comprising the US and Canada, North America held the largest market share. This region dominates the global clinical trial support services market due to its prominent medical biotechnology industry, robust regulatory framework, high investment in R&D, and extensive experience in conducting clinical trials across various medical centers. The presence of large contract research organizations (CROs), academic institutions, and healthcare facilities, along with advanced healthcare systems and a sound payment infrastructure, contributes to the region’s leadership. The adoption of new technologies, such as telemedicine, has also increased clinical trial activity in North America.
Europe: Europe is a key market for clinical trial support services, characterized by a stable regulatory environment, well-established research infrastructure, and a highly skilled workforce. The region has a rich history of clinical research, supported by industry, educational, and governmental agencies. Countries like France serve as major hubs for clinical trials, offering diverse patient populations, advanced hospitals, and experienced researchers. Policies and initiatives from the European Medicines Agency (EMA), such as the Innovative Medicines Initiative (IMI), encourage innovation and expedite the development of new treatments.
Asia-Pacific: This region is emerging as an attractive market for clinical trial support services, driven by rapid economic growth, rising healthcare spending, and large patient cohorts. China, Japan, and India are experiencing increased clinical trial activity, facilitated by government policies aimed at attracting foreign investment, streamlining regulatory processes, and enhancing research infrastructure. The region offers advantages such as lower operating costs, faster patient recruitment, and access to diverse ethnic populations. However, challenges like cultural and regulatory differences, data privacy concerns, and intellectual property issues remain significant considerations for sponsors and service providers operating in the area.
Rest of the World: This segment includes emerging markets in Latin America, the Middle East, and Africa, which present untapped opportunities for clinical trial support services. Despite infrastructural, regulatory, and social challenges, strategic partnerships, capacity building, and technology transfer programs are facilitating access to clinical research capabilities in these regions. These efforts attract sponsors and CROs looking to expand their global footprint and reach new patient populations.
Browse In-depth Market Research Report (128 Pages) on Clinical Trial Support Services: https://www.marketresearchfuture.com/reports/clinical-trial-support-services-market-22002
Competitive Dynamics
MRFR recognizes the following companies as the key players in the global clinical trial support services market— Charles River Laboratories International, Inc., Wuxi Apptec, Inc., Iqvia Holdings, Inc., Syneos Health, Inc., Eurofins Scientific, Ppd, Inc. (Pharmaceutical Product Development), Icon Plc, Laboratory Corporation Of America Holdings (Labcorp), Alcura, Parexel International Corporation.
Discover More Research Reports on Healthcare Industry by Market Research Future:
Homeopathic Medicine Market Research Report Information By Type (Plant-Based, Animal-Based, and Minerals-Based), By Application (Reproductive Disorders, Hormonal Imbalance, and Lifestyle Diseases), By End-Users (Hospitals and Homeopathic Clinics), And By Region (North America, Europe, Asia-Pacific, And Rest Of The World) –Market Forecast Till 2032.
Contact Lenses Market Research Report Information By Type (Corrective Lens, Therapeutic Lenses, and Cosmetic & Lifestyle Oriented Lens), By Wear Type (Daily Disposable Lenses, Disposable Lenses (Two Weeks or Sooner), Frequent Replacement Lenses (Monthly or Quarterly), and Conventional Lenses), By Material (Methacrylate Hydrogel Soft Contact Lens, Silicone Hydrogel Soft Contact Lens, Gas-Permeable Contact Lens, and Others), By Design (Spherical Lens, Toric Lens, Multifocal Lens, and Others) And By Region.
In Vitro Fertilization Market Research Report Information By Type (Intrauterine Insemination, Intracytoplasmic Sperm Injection, Ivf Using Donor Eggs), By Devices (Imaging Systems, Sperm Seperation System, Ovum Aspiration Pump, Micromanipulator, Cryosystem), By Reagents (Embryo Culture Media, Cryopreservation Media, Sperm Processing Media, Ovum Processing Media), By End Users (Clinical Research Institutes, Fertility Clinics, Hospitals), And By Region (North America, Europe, Asia-Pacific, And Rest Of The World) – Market Forecast Till 2032.
Cord Blood Banking Services Market Research Report Information by Storage Service (public cord blood banks, private cord blood banks, and hybrid cord blood banks), by Component (Cord blood and cord tissue), by Application (cancer, blood disorders, metabolic disorders, immune disorders, and others), by End User (hospitals, pharmaceutical research, and research institutes), and By Region (North America, Europe, Asia-Pacific, And Rest Of The World) – Forecast Till 2032.
US Aesthetics Market Research Report Information By Product (Facial Aesthetic Products, Cosmetics Implant, Skin Aesthetic Devices, Body Contouring Devices, Physician-Dispensed Cosmeceuticals & Skin Lighteners, Hair Removal Devices, Tattoo Removal Devices, Thread Lift Products, Physician- Dispensed Eyelash Products, and Nail Treatment Laser Devices), By Procedure (Surgical Procedures {Breast Augmentation, Rhinoplasty, Facelift & Body Lift, and Other Surgical Procedures}, and Non-Surgical Procedures.
About Market Research Future:
Market Research Future (MRFR) is a global market research company that takes pride in its services, offering a complete and accurate analysis with regard to diverse markets and consumers worldwide. Market Research Future has the distinguished objective of providing the optimal quality research and granular research to clients. Our market research studies by products, services, technologies, applications, end users, and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help answer your most important questions.
Follow Us: LinkedIn | Twitter
Contact:
Market Research Future (Part of Wantstats Research and Media Private Limited)
99 Hudson Street, 5Th Floor
New York, NY 10013
United States of America
+1 628 258 0071 (US)
+44 2035 002 764 (UK)
Email: [email protected]
Website: https://www.marketresearchfuture.com
Contact Information:
99 Hudson Street, 5Th Floor New York, NY 10013 United States of America +1 628 258 0071 (US) +44 2035 002 764 (UK) Email: [email protected] Website: https://www.marketresearchfuture.com
Tags:
Research Newswire, English




