The Transseptal Access Systems Market To Move Across The Exalted Enigma Backed By Innovation
iCrowdNewswire
Nov 09, 2021

The global Transseptal Access Systems Market is expected to be on a splendid growth spree In Upcoming Years. Every vertical is onto the creation of a talent pool to cater to the demands from the public as well as the private sector. Moreover, the emphasis lays on a digital pool of ready-to-hire, experienced, and highly skilled professionals. With close to 10K investment opportunities over the next 10 years, the future is there to witness an escalation herein.
Surging obese population, increasing elderly population with greater risk of cardiac diseases, and rapid technological advancements in transseptal access systems are key factors contributing to significant increase in their sales. For example, the industry has seen the introduction of advanced technologies such as left atrial appendage occlusion therapy for patients with high risk of stroke due to non-valvular atrial fibrillation (AF). Such developments reduce morbidity and enhance quality of life, as well increase survival rate.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/18703
Market players are following several marketing strategies such as increasing sales forces, increasing distribution channel, product launching, and acquisitions.
- In 2012, Cook Medical opened a distribution center in Baesweiler, Germany. This expansion has resulted in increasing penetration in the European market.
- In 2016, Terumo signed an agreement with Abbott Laboratories to acquire Vado Steerable Sheath.
Company Profiles:
- Terumo Corporation
- Medtronic PLC
- St. Jude Medical (Abbott Laboratories)
- Boston Scientific
- Biosense Webster, Inc. (Johnson & Johnson)
- Merit Medical Systems, Inc.
- Baylis Medical Company Inc.
- Pressure Products Medical Device Manufacturing LLC
- Cook Medical LLC
- Transseptal Solutions, Inc.
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/18703
Key Takeaways from Market Study
- Based on product, transseptal access sheaths are leading with over 86% market share.
- Atrial fibrillation ablation is estimated to dominate the market by application. This segment accounted for approximately 65% share of the market, primarily due to rise in the prevalence of atrial fibrillation.
- Hospitals dominate with a market share of 49%. Incidence of atrial fibrillation-related cases is rising dramatically, which has led to a rise in the adoption of these therapies by various hospitals.
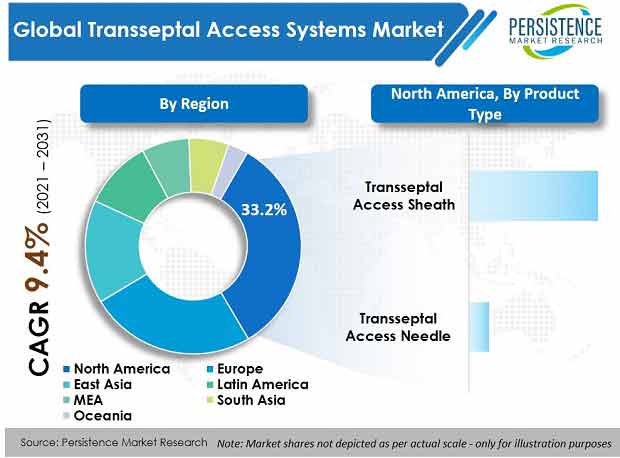
- By region, North America is set to dominate the global transseptal access systems market with a value share of around 33%.
- Europe is slated to be the second-largest market with a value share of 25% during the forecast period.
“Launch of new product lines and continuous improvements in available transseptal access systems have enhanced the safety and efficacy of these systems,” says an analyst of Persistence Market Research.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/18703
Market Competition
New product launches is the winning strategy used by leading manufacturers of transseptal access system. Continuous technological advancements and innovation in transseptal access systems are accountable for intense competition among key players. Several organizations are focusing on new product approvals and launches for transseptal access systems.
- For instance, in Jan 2019, Baylis Medical launched ExpanSure, a transseptal access system that combines dilators and sheaths into a single device, which assists in larger dilation as compared to fixed curve sheaths.
- In 2018, Transseptal Solutions Ltd got U.S. FDA approval for the TSP Crosser Transseptal Access System, which is aimed to deliver steady catheter placing and meticulous access.
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the Transseptal Access Systems Market in its latest study, presenting a historical demand assessment of 2016 – 2020 and projections for 2021 – 2031.
The research study is based on the product {Transseptal Access Needle (Standard Curve (C0) Needle and Large Curve (C1) Needle) and Transseptal Access Sheath (Steerable Sheath and Fixed Sheath)}, application (Atrial Fibrillation Ablation, Mitral Valve Repair and Left Atrial Appendage Occlusion), and end user (Hospitals, Ambulatory Surgical Centers and Specialty Clinics), across seven key regions of the world.
About Us: Persistence Market Research
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – [email protected]
Website – https://www.persistencemarketresearch.com




