FDA Clears First Medical Device Accessory for Apple Watch®
Press Releases
Nov 30, 2017
MOUNTAIN VIEW, Calif., Nov. 30, 2017 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (EKG) technology, today announced FDA clearance of KardiaBand in the U.S., allowing Apple Watch users to discreetly capture their EKG anytime, anywhere in order to quickly detect normal sinus heart rhythms and atrial fibrillation (AFib), the most common heart arrhythmia. The first FDA-cleared medical device accessory for Apple Watch, KardiaBand can record an EKG in 30 seconds with just a touch of its integrated sensor. Results from the Kardia App are displayed on the face of Apple Watch.
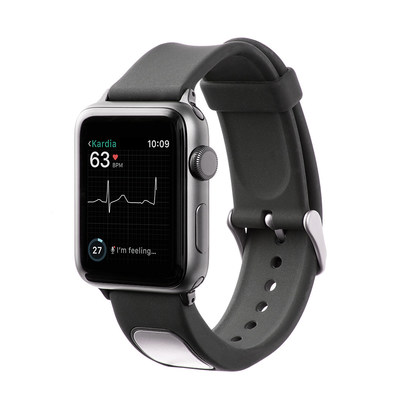
AliveCor is also introducing SmartRhythm, a new feature within the Kardia app for Apple Watch. SmartRhythm uses artificial intelligence in concert with inputs from Apple Watch’s heart rate and activity sensors to continuously evaluate the correlation between heart activity and physical activity. When SmartRhythm detects that heart rate and activity are out of sync, the device notifies users to capture an EKG with KardiaBand, or with KardiaMobile, AliveCor’s popular, portable EKG reader.
“KardiaBand paired with SmartRhythm technology will be life-changing for people who are serious about heart health,” said Vic Gundotra, CEO, AliveCor. “These capabilities will allow people to easily and discreetly check their heart rhythms when they may be abnormal, capturing essential information to help doctors identify the issue and inform a clear path of care to help manage AFib, a leading cause of stroke, and other serious conditions.”
Atrial Fibrillation (AFib), is the most common heart arrhythmia, and a leading cause of stroke. AFib affects more than 30 million people worldwide, and one in four people over the age of 40 are at risk for developing it. Millions of people around the world are unknowingly living with AFib. Yet, two out of three strokes are preventable when AFib is detected and treated appropriately.
“This is a paradigm shift for cardiac care as well as an important advance in healthcare,” said Dr. Ronald P. Karlsberg, MD FACC, Board Certified Cardiologist and Clinical Professor of Medicine, Cedars Sinai Heart Institute and David Geffen School of Medicine UCLA. “Today, EKGs are available only in offices and hospitals, using complex equipment, and usually only after a life threatening event, for example a stroke. With an EKG device on the wrist, AFib can be detected wherever the patient is, 24 hours a day. In randomized research trials, KardiaMobile, the first AliveCor EKG device, proved to be superior to routine care provided by physicians. Today, KardiaBand is a giant leap in personalized health care.”
As a medtech leader, AliveCor uses advanced artificial intelligence, mobile, cloud and micro-electrode technology to change the dynamic in cardiac care. AliveCor empowers people worldwide to proactively manage heart health and to vastly improve the quality of care in the fight against heart disease. AliveCor’s KardiaMobile and KardiaBand enable people and their care teams to easily, quickly and inexpensively detect and manage possible abnormal heart rhythms.
KardiaBand is available starting today for $199 and requires subscription to AliveCor’s Premium service for $99 a year. The combined system includes SmartRhythm notifications on Apple Watch; unlimited EKG recordings; automatic detection of Atrial Fibrillation or normal sinus rhythm; the unlimited ability to send EKG readings to anyone via email; unlimited cloud history and reporting of all EKGs ever taken; weight and medication tracking; and a mailed monthly paper report on readings taken each calendar month.
About AliveCor
AliveCor, Inc. is pioneering the creation of FDA-cleared machine learning techniques to enable proactive heart care and is recognized around the world for transforming cardiac care. The FDA-cleared KardiaMobile is the most clinically validated mobile EKG solution on the market. It is recommended by leading cardiologists and used by people worldwide for accurate EKG recordings. KardiaMobile, and KardiaBand, when paired with the Kardia app provide instant analysis for detecting atrial fibrillation (AF) and normal sinus rhythm in an EKG. Kardia is the first A.I. enabled platform to help clinicians manage patients for the early detection of atrial fibrillation, the most common cardiac arrhythmia and one that leads to a five times greater risk of stroke. KardiaBand is the first FDA-cleared medical device accessory for Apple Watch. AliveCor was recognized by Fast Company as one of 2017’s most innovative companies in health (#3). AliveCor is a privately-held company headquartered in Mountain View, Calif. For more information, please visit alivecor.com.
For more information on partnership inquiries, please contact the following:
US: [email protected]
International: [email protected]
Media Contact: Morgan Mathis at Highwire PR for AliveCor
[email protected]

View original content with multimedia:http://www.prnewswire.com/news-releases/fda-clears-first-medical-device-accessory-for-apple-watch-300564129.html
SOURCE AliveCor


