CurePSP & Dthera Sciences Announce Initial Positive Results of Pilot Program
Press Releases
Oct 04, 2017
SAN DIEGO, Oct. 4, 2017 /PRNewswire/ — Dthera Sciences (OTCQB:DTHR), a digital therapeutics company focused on developing innovative digital quality of life therapies for neurodegenerative diseases and oncology, announced today the positive initial results of a pilot Program with CurePSP, a leading nonprofit focused on neurodegenerative diseases.
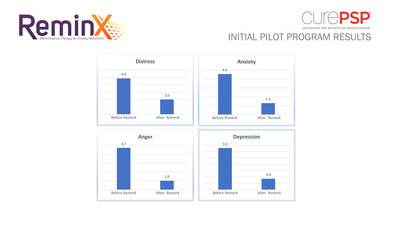
CurePSP and Dthera recently engaged in a pilot program in which individuals diagnosed with PSP used ReminX, Dthera’s leading digital therapeutic designed to reduce anxiety and improve quality of life. The initial results for PSP were decidedly positive and thus, CurePSP and Dthera have agreed to work together to make the ReminX product available for the wider CurePSP community with the hope of gathering additional information as well as making a positive impact in the quality of life in as many PSP patients and their families as possible.
The pilot program tested several factors to determine the effectiveness of ReminX. Caregivers asked PSP patients how they felt before and after viewing ReminX, including a score from 1-10 indicating how distressed, anxious, depressed and angry patients felt. On average, there was a reduction of all symptoms. The product was used in an acute setting and results in a chronic setting are not yet known.
“I am very encouraged with the initial findings from the pilot program and believe that through working together with CurePSP, we can make ReminX available to more members of the PSP community and continue to gather critical data on how to make the most significant positive impact for those suffering from neurodegenerative diseases,” said Dthera Sciences CEO, Edward Cox.
David Kemp, President of CurePSP, added, “We are delighted to be working with Dthera Sciences on this exciting project, as it offers the promise of improving the lives of patients and families suffering from PSP and related diseases. While these diseases are fatal and largely untreatable, ReminX holds the promise of greatly improving quality of life while we search for a cure.”
About CurePSP
CurePSP is the leading nonprofit advocacy organization focused on prime of life neurodegenerative diseases – a spectrum of fatal brain disorders that often strike during a person’s most productive and rewarding years. Currently there is no treatment or cure for these diseases, which affect more than 150,000 people in the U.S. alone. Since it was founded in 1990, CurePSP has funded more than 180 research studies and is the leading source of information and support for patients and their families, other caregivers, researchers and doctors and allied healthcare professionals. CurePSP is based in New York City. Please visit www.curepsp.org for more information.
About Dthera Sciences
Dthera Sciences, based in San Diego, CA, is a digital therapeutics company focused on developing innovative digital ‘quality of life’ therapies for neurodegenerative diseases and oncology. The Company’s lead product, ReminX, is an artificial-intelligence-powered digital therapeutic designed to reduce anxiety and improve quality of life in patients with Alzheimer’s disease and Dementia. For more information, please visit www.dthera.com and www.reminx.com
About Digital Therapeutics
Digital Therapeutics is a new subsection of digital health that strives to directly deliver a therapy via use or interaction with software technology. The goal of Digital Therapeutics is to mirror an effective treatment and use technology to scale it to a larger patient population, thereby amplifying doctors’ and nurses’ care, changing patient behavior, and most importantly, reducing cost of care.
Forward Looking Statement
This press release contains “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995, regarding the research, development and commercialization of therapeutic products and technologies, as well as the Company’s efforts to increase its customer base and initiate additional clinical trials. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert or change any of the statements made, and could cause actual outcomes and results to differ materially from current expectations. No forward-looking statement can be guaranteed. These forward-looking statements are made as of the date of this press release, and the Company expressly disclaims any intention or obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements. Readers are urged to read the risk factors set forth in the Company’s most recent annual report on Form 10-K, subsequent quarterly reports filed on Form 10-Q, and other filings made with the SEC. Copies of these reports are available from the SEC’s website at www.sec.gov or without charge from the Company.
CONTACT INFORMATION
Geno Kostikov
Director, Product & Corporate Development
Dthera Sciences
[email protected]
(858) 215-6360
David Kemp
President
CurePSP
[email protected]
(802) 734-1185
View original content with multimedia:http://www.prnewswire.com/news-releases/curepsp–dthera-sciences-announce-initial-positive-results-of-pilot-program-300530692.html
SOURCE Dthera Sciences



